BOM Sampling Plan
Sampling is a method widely used to define product meeting Quality Criteria.
What is Sampling?
Sampling is the selection of a representative portion of the inventory of an item to make inferences about the entire inventory. In pharmaceutical manufacturing, samples are drawn from different stages of the process for both controlling process parameters and assessing product quality.
Why Sampling?
Instead of waiting until production is in full swing, the sampling process allows you to catch discrepancies or failures to meet your specification standards early on in the process. This can prevent a large number of wasted resources, including both time and money.
How can I set Sampling in DataNinja?
Within the following sections, we will explain to you how to set your sampling plan as part of the process when creating your Manufacturing Master Record.
Acceptable Quality Level (AQL)
DataNinja uses AQL "General Inspection Levels" as the parameter, and these values are already integrated into the system as pre-existing values. What this means is that DataNinja will do the math based on your:
- Lot size (quantity)
- Inspection levels/sampling plan (i.e. Reduced I, Normal II, or Tightened III).
- Defect classifications (i.e. Critical, Major, or Minor).
- AQL level corresponding to inspection & defect levels.
The user will be guided by the system (DataNinja) to complete the inspection process as soon the production starts manufacturing.
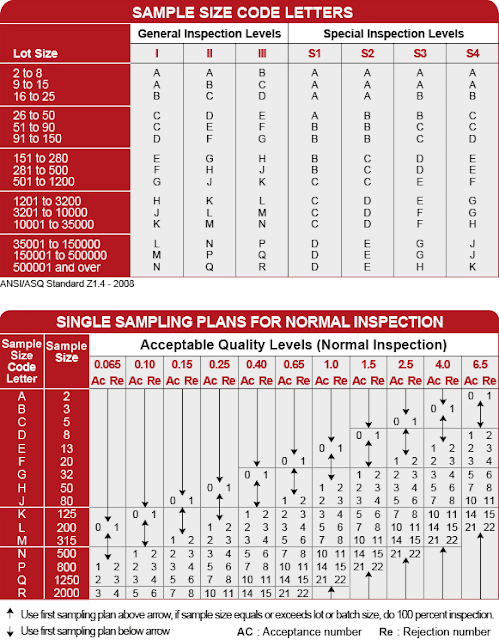
Based on the information above, a plan can be generated for sampling and acceptance limit.
Example:
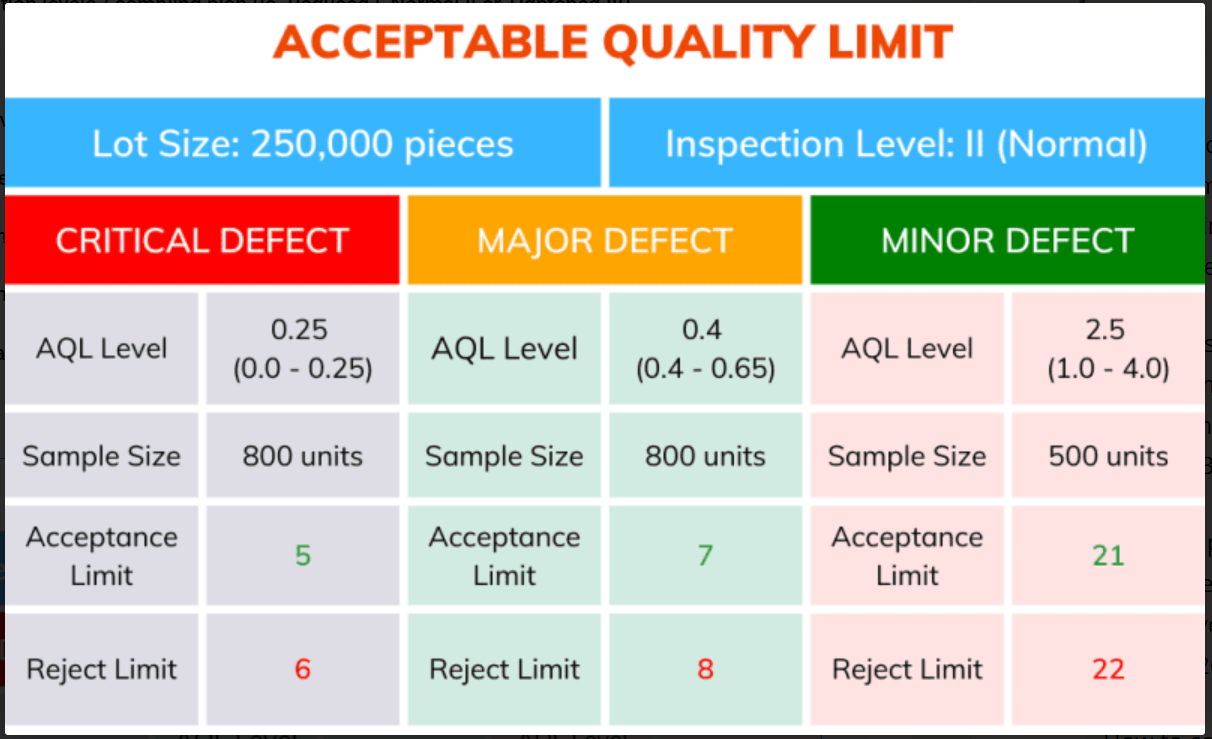
Setting Sampling Plan in Data Ninja
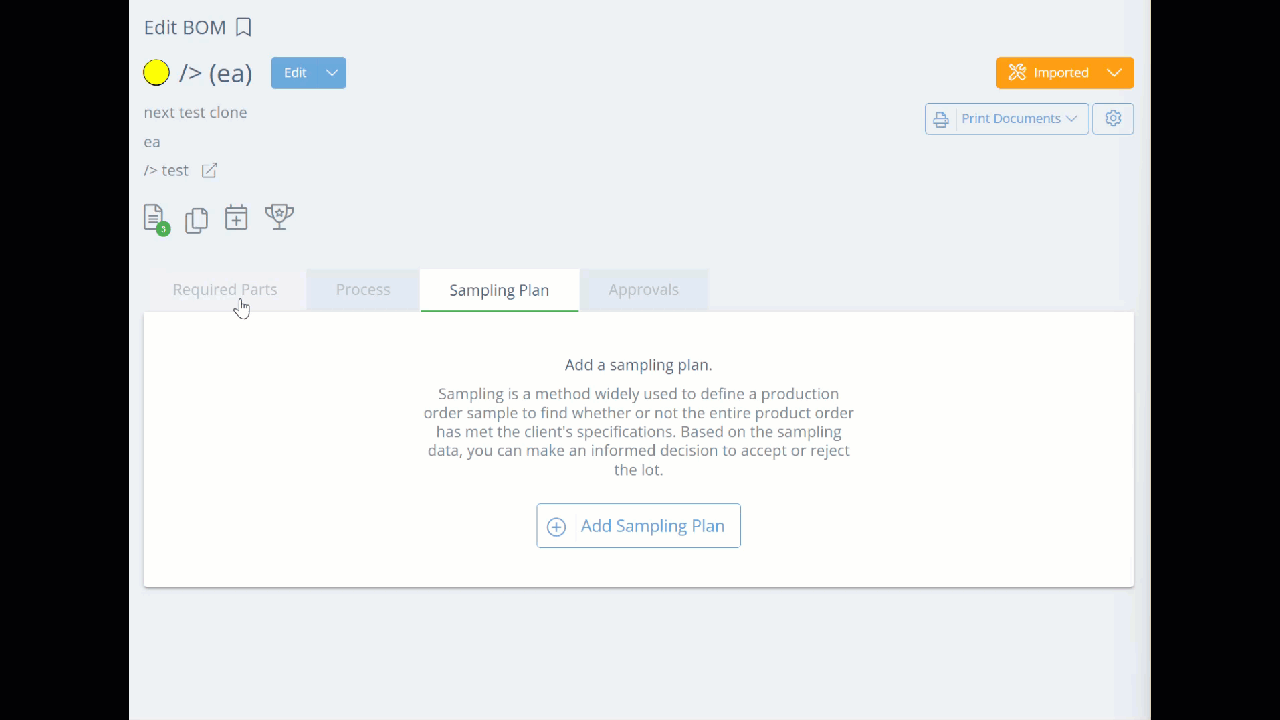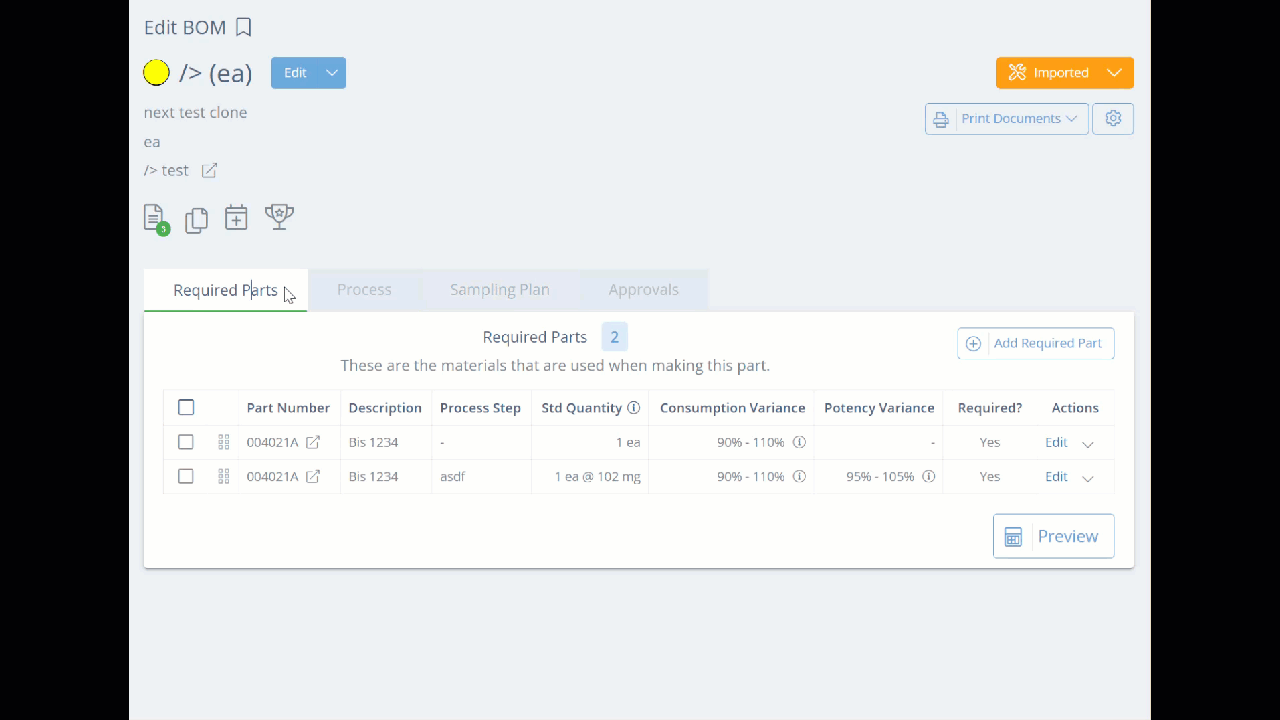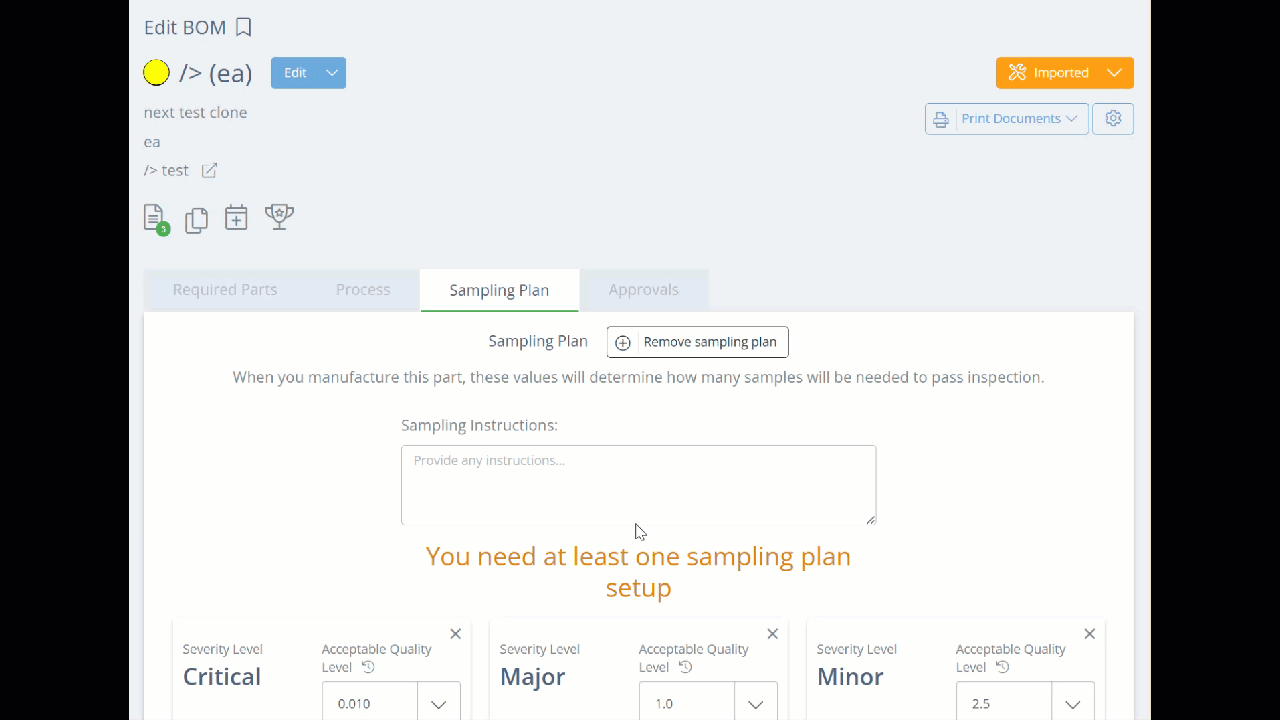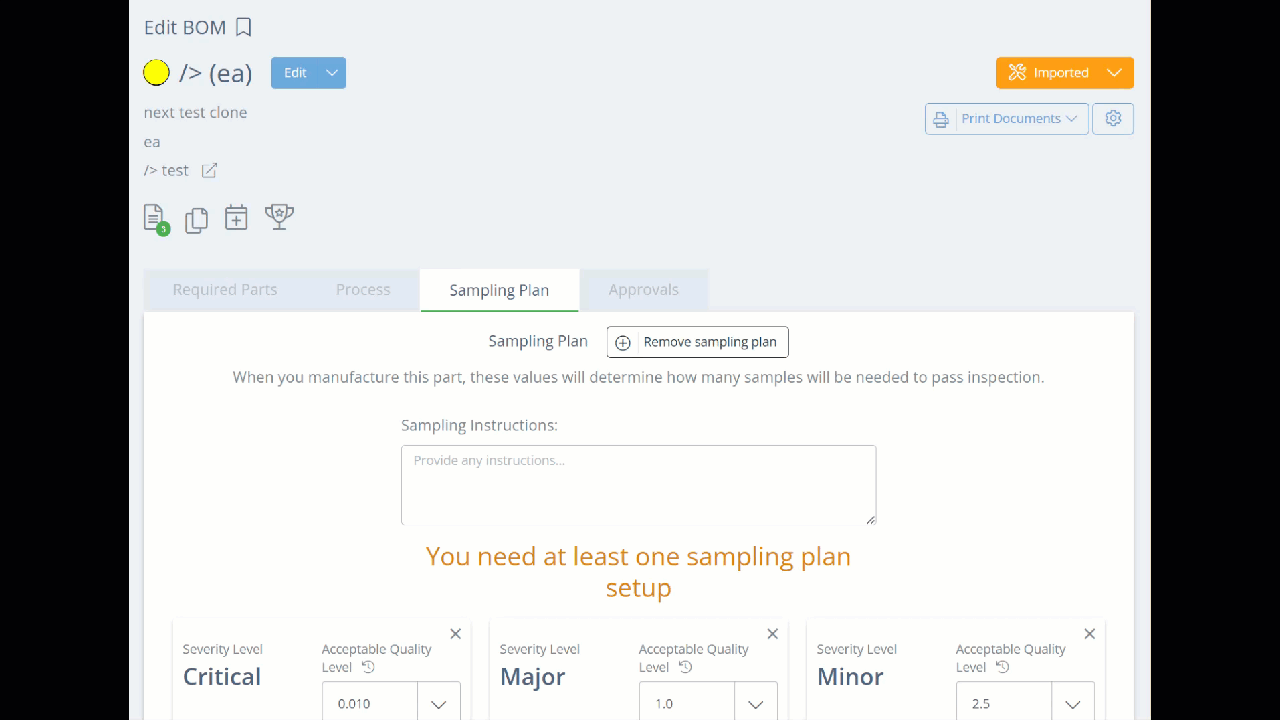
To proceed and add the sampling plan, go to the corresponding Bill of Materials, and click the Sampling Plan tab, then click the "Add Sampling Plan" button.
Set AQL Levels and Add Defect Categories
Click "Add Sample Plan" and select the plan you will be using, based on your company regulations. Severity levels vary depending on the business of the company.
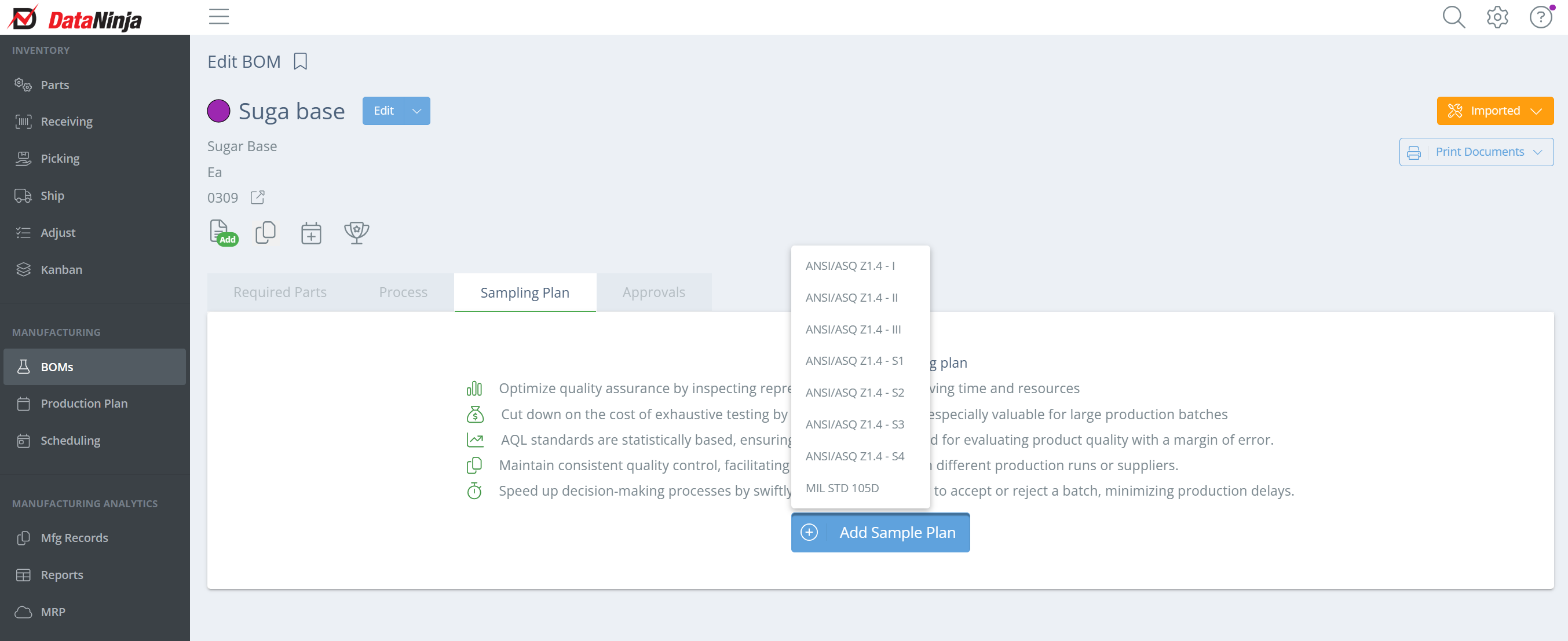
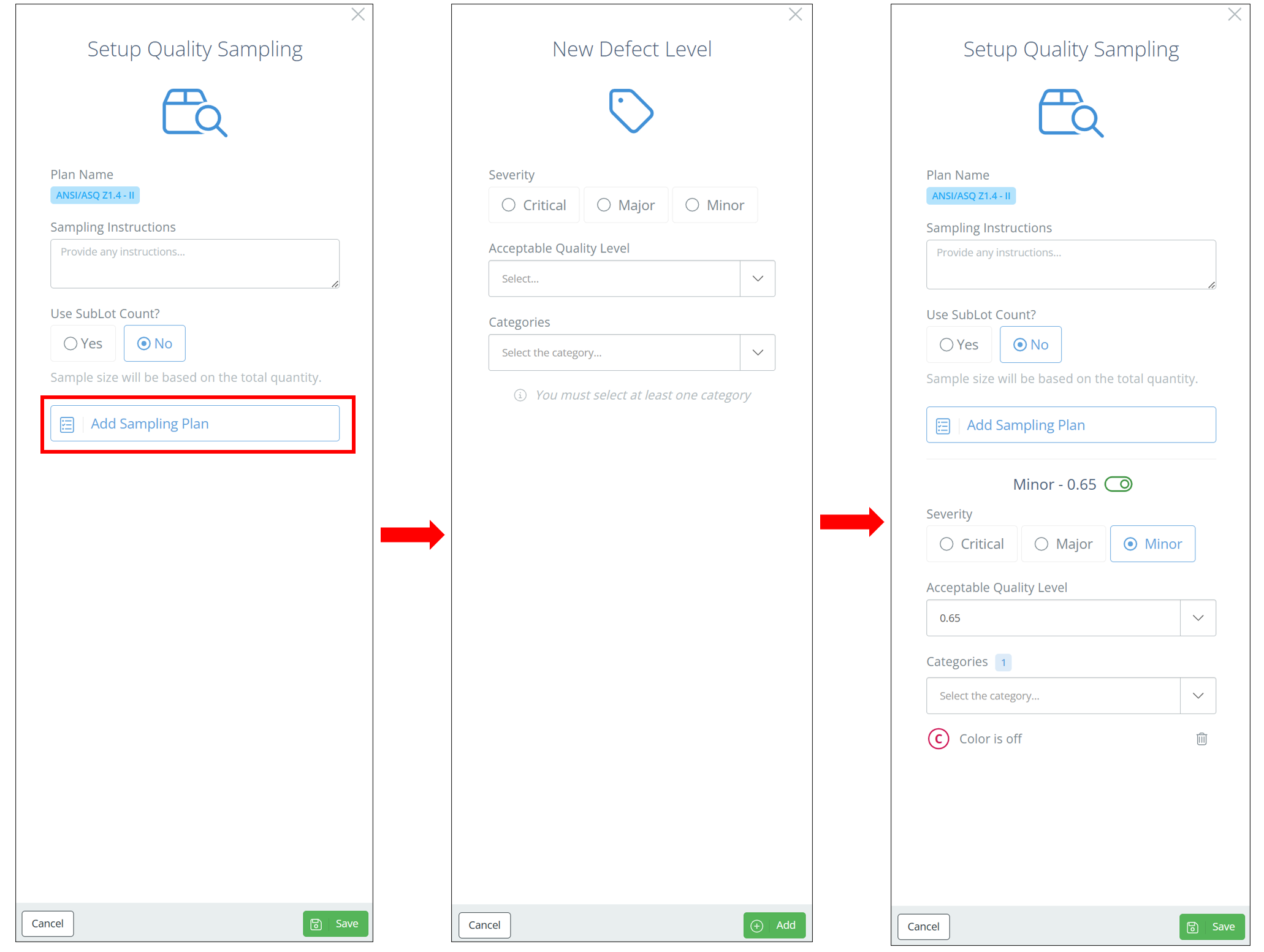
After choosing the sampling plan, a new sidebar should show on the right-hand side of the screen. List any sampling instructions and specify whether you want to use sublot count.
Sublot Count or Not?If you select "Yes" to sublot count, the sample size will be based off the number of labels. If you select "No" then the sample size will be based on the total quantity produced in a batch instead. Choose whichever option best fits your needs and sampling requirements.
Add Sampling Plan
Defects are required to be categorized depending on companies' regulations. For example, the "Critical" defects category encompasses all defects that can be hazardous or unsafe for the individuals using/consuming them.
To add these defects, click "Add Sampling Plan" and select a defect severity level. Specify the AQL and any categories, then click "Add". Once all desired severity levels have been added, click "Save" and the sampling plan will show on the Edit BOM page.
Can I update my sampling plan?Yes, you can update a sampling plan until the BOM is released and scheduled. This action will lock the Manufacturing Master Record and can only be updated by revising the MMR. Use our Copy a BOM page for directions.
Updated 4 months ago
